how many valence electrons does mercury have|How Many Valence Electrons Does Mercury Have? : Bacolod By Staff WriterLast Updated December 11, 2023. Mercury has two valence electrons, both of which sit in the atom’s 6s shell. A valence electron is an electron in .
Synonyms for FAVOR: kindness, service, indulgence, good turn, grace, approbation, befriending, favour; Antonyms for FAVOR: disfavor, opposition, disapproval, dislike, misprize, disesteem, abuse, bear a grudge against.
how many valence electrons does mercury have,Mar 23, 2023
The web page lists the valences of the elements from hydrogen to thallium, . The valency of mercury is 2 since it has the 2 valence electrons in its outer shell. Valency is the combining capacity of mercury or any other chemical element . . How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so .

Mercury is a Transition Metal element. It is part of group 12 (zinc family). Know everything about Mercury Facts, Physical Properties, Chemical Properties, Electronic configuration, Atomic and Crystal Structure. . By Staff WriterLast Updated December 11, 2023. Mercury has two valence electrons, both of which sit in the atom’s 6s shell. A valence electron is an electron in .
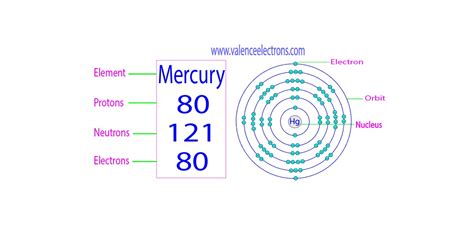
Origin of the name. Mercury is named after the planet, Mercury. Allotropes. Hg. Mercury. 80. 200.592. Glossary. GroupA vertical column in the periodic table. Members of a group .Elements. Mercury (Hg) Mercury is a chemical element of the periodic table with chemical symbol Hg and atomic number 80 with an atomic weight of 200.592 u and is classed as .Mercury has an atomic number of 80, indicating the presence of 80 protons and 80 electrons in a neutral atom, and an atomic weight of 200.59 g/mol. Mercury is located .
Mercury has an atomic number of 80, indicating the presence of 80 protons and 80 electrons in a neutral atom, and an atomic weight of 200.59 g/mol. Mercury is located in period number 6, group number 12, and is a d-block transitional metal with an Electron configuration [Xe] 4f14 5d10 6s2 and two valence electrons. Mercury is a liquid at room .Mercury. 80. 200.592. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2) In fact, the number of valence electrons goes up by one for each step across a . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
To find the number of valence electrons for Gold (Au) we need to look at its electron configuration. This is necessary because Au is a transition metal (d b.
how many valence electrons does mercury have How Many Valence Electrons Does Mercury Have? To find the number of valence electrons for Gold (Au) we need to look at its electron configuration. This is necessary because Au is a transition metal (d b.How Many Valence Electrons Does Mercury Have? Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .how many valence electrons does mercury have Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .What is the nobel gas configuration? How many valence electrons are in the ground state electron configuration of mercury? Given: atomic number. Asked for: complete electron configuration. Strategy: Using the orbital diagram in Figure 6.8.1 and the periodic table as a guide, fill the orbitals until all 80 electrons have been placed. Solution: How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Neon, with its configuration ending in .
How many valence electrons does a Mercury atom have? Mercury has 2 valence electrons. Mercury has 80 electrons out of which 2 valence electrons are present in the 5d10 6s2 outer orbitals of atom. What is the melting Point of Mercury? Melting Point of Mercury is 234.32 K.

Mercury is a chemical element of the periodic table with chemical symbol Hg and atomic number 80 with an atomic weight of 200.592 u and is classed as a transition metal. . Valence electrons : 2: Valency electrons : 1,2: Bohr model: Electron shell for Mercury, created by Injosoft AB Hg. Figure: Shell diagram of Mercury (Hg) atom. Orbital .
Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals. However, this is an incorrect perspective, as quantum mechanics demonstrates that electrons are more complicated. Figure 2.6.1 2.6. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball! Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable . In this video we will write the electron configuration for Mercury (Hg) and the Mercury (II) ion. We’ll also look at why Mercury forms a 1- ion and how the e. How many d electrons does Hg have? Mercury (the neutral atom) has 8o electrons. How many electrons does mercury have in its outer level? 2. . Mercury easily shares its valence electrons. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Mercury have? In the case of Mercury the valence electrons is 1,2.
As we move down in a group the number of valence electrons does not change. Hence, all the elements of a particular group group have the same valency. . What is the Valency of Mercury? Reply. Mentor. July 16, 2020 at 11:39 am. The Valency of Mercury is 2, 1. Reply. Dhanya. July 30, 2020 at 11:38 pm. Good it very helpful. Reply.
how many valence electrons does mercury have|How Many Valence Electrons Does Mercury Have?
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons Chart for All Elements
PH2 · Mercury Valence Electrons
PH3 · Mercury (Hg)
PH4 · Mercury
PH5 · How Many Valence Electrons Does Mercury Have?
PH6 · Determine valence electrons using the periodic table
PH7 · 10.6: Valence Electrons